BACKGROUND
If the needle is misaligned with the target during the insertion task, the physician can either re-attempt the insertion, or manipulate the exposed rear part of the needle to create a corrective bending moment. The latter method, base manipulation, is often practiced as new insertion attempts increase the risk of complications (Kratchman et al. 2011). However, base manipulation suffers from a depth dependency as the moment arm decreases and the tissue resistance increases with insertion depth (Reed et al. 2008), hampering needle tip placement at larger depths.
Accurate needle positioning remains challenging in clinical situations, not only in prostate cancer cases, but in any biopsy or treatment that requires accurate positioning. Needles used in interventional procedures are easily affected by external (environmental) factors such as tissue inhomogeneity and boundary layers as they become thinner and more flexible (van Gerwen et al. 2014). In case of needle interventions in deep-seated tissue structures, this leads to difficulties with regard to target alignment. Resulting targeting errors may reduce the efficiency and reliability of image guided biopsy for diagnosis of suspicious lesions or therapy. In general, errors arise from human factors, imaging limitations, and dynamic needle-tissue interactions, including soft tissue deformations, needle deflections and sliding of multi-layered soft tissue (Gao et al. 2012). Especially for needles with an asymmetrically bevelled tip, significant placement errors can occur (Blumenfeld et al. 2007, Roesthuis et al. 2013). In addition, target movements due to physiological processes, e.g. breathing (Zhou et al. 2012) or heartbeat, inherently introduce placement difficulties. Consequences of tip misplacement may be additional damage due to re-puncturing (Kratchman et al. 2011), misdiagnosis (false negatives), poor dosimetry, and tumor seeding (DiMaio et al. 2003).
Recent approaches to improve the targeting accuracy in percutaneous interventions include path planning based on models that predict tissue deformation and needle deflection (DiMaio et al. 2005, Misra et al. 2010) and the use of robotic systems for steering the needle (Glozman et al. 2007, Gao et al. 2012). Steerable needles allow for active control of the needle trajectory and can compensate for target movement and needle deflection during insertion (Henken et al. 2013). Additionally, delicate tissue (e.g. nerves or vessels) can be circumvented and deep structures can be accessed. Well-studied principles in needle steering include steering with bevel or pre-curved needle tips (Majewicz et al. 2012), and active cannula mechanisms (Dupont et al. 2010). The applicability of these robotic systems depends on accurate control based on predictive models (Goa et al. 2012) that describe the interaction between the needle and tissue. However, as the mechanical properties are generally unknown (van Gerwen et al. 2012) there are yet no clinically applicable solutions available.
STATE OF THE ART
In terms of approach, needle tip placement techniques can be divided in initial aligning and subsequent correction or steering tasks. Prepuncture alignment can be robotically assisted. Insertion after needle alignment is either performed manually (Solomon et al. 2006) or automatically (Zhou et al. 2013) under image guidance. Here we will focus on the state of the art with respect to insertion and steering after the initial alignment has been completed.
Needle steering can be divided into passive and active techniques. In both cases, the needle cannula will eventually bend. During passive needle insertion, bending forces are a direct result of the needle–tissue interactions. For active needles, either the tip or the cannula shape can be modified without tissue contact. During insertion in tissue, this modification typically also translates to a cannula deformation.
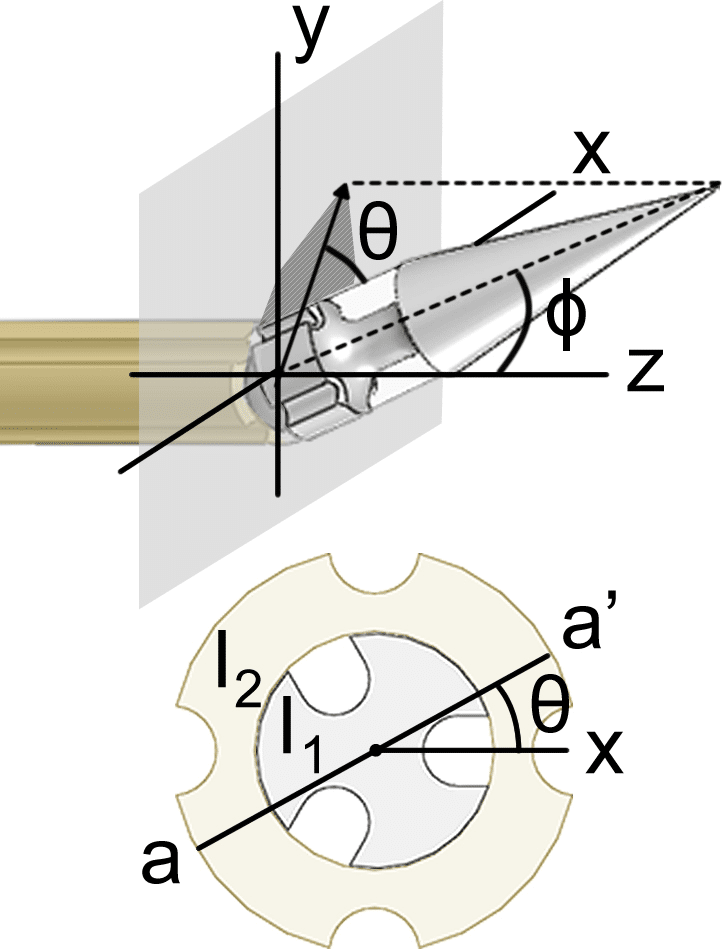
Here we distinguish six steering techniques, as shown in Fig. 1. This figure also presents the degrees of freedom used in needle control. These steering techniques are, respectively, base manipulation, bevel tip steering, precurved stylet steering, active cannula (AC) steering, programmable bevel steering, and tendon actuated tip steering.
It is important to note that during passive steering, cannula deformations result from interactions with surrounding tissue. During active steering, either the tip or cannula can, to some extent, be deformed without making tissue contact. Needle steering is constrained by both the navigation medium and the needle itself, including both mechanical design and operational factors, like flexural rigidity and insertion speed. In practice, the navigation medium is highly variable or unknown. In this regard, it has been regularly acknowledged that the insertion success rate can be low with current steerable needle designs (Rucker et al. 2015, Adebar et al. 2015). Possibly the stiffness of clinically applied needles could serve as a guide for future designs.
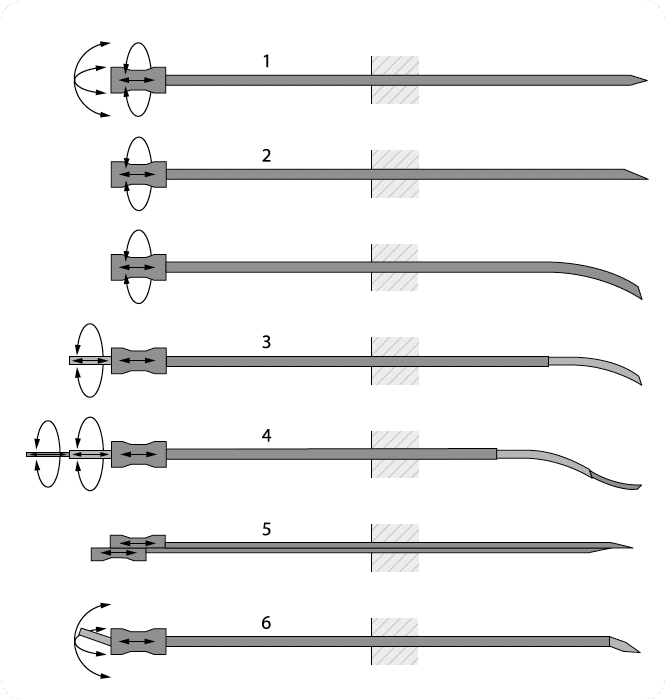
The depicted techniques are, respectively:
- (1) base manipulation,
- (2) bevel tip (with and without precurve),
- (3) precurved stylet,
- (4) Active cannula,
- (5) programmable bevel,
- (6) tendon actuated tip steering.
The programmable bevel is here presented with two segments, versions with four segments are available as well.
STEERABLE NEEDLES FOR MR GUIDED INTERVENTIONS
Next to adequate steering possibilities, adequate visualization of the instrument and the target anatomy is paramount to accurate targeting in minimally invasive needle interventions. Though several advances have been made in external tracking systems, such as improvements in accuracy, and novel field generators that have shielding preventing influence of large metal parts (Yaniv et al. 2009), or can attached to the ultrasound transducers (Marz et al. 2014), we opt for an image-based approach of needle tracking. Such an approach does not require an active sensor, or external tracking system. The latter is relevant in an interventional environment that is already cluttered by various systems. Furthermore, the needle is tracked in the same image that is being used for locating the target, and thus the needle position is intrinsically registered to the patient. It thus is an approach that is easy to translate to current clinical practice.
Imaging modalities that are generally being used to guide needle insertions are CT, X-ray and Ultrasound.
- CT image guidance provides 3D images, of relatively good quality; however, CT is also relatively expensive, uses ionizing radiation and is not real-time.
- X-ray is real-time, but often lacks sufficient soft tissue contrast that is required for needle introductions.
- MR imaging therefore is often considered the preferred modality for image guided needle interventions:
It is real-time, provides soft tissue contrast, and currently also is available as a 3D imaging modality.
This latter option is required if a curved needle needs to be imaged. Given the needle tip position and orientation, visualizations such are commonly used for 3D image guided procedures (i.e. parallel to the needle, cross-sectional to the needle, in front of the needle tip) can be easily generated and displayed.
Currently, the number of clinically performed MR guided interventions is small relative to the overall number of percutaneous interventions, either performed under CT guidance or with the help of ultrasound imaging. Also, the number of available devices suitable for use in the MR is limited. Here we show some recently developed steerable needles that in principle could be used in the MR setting.
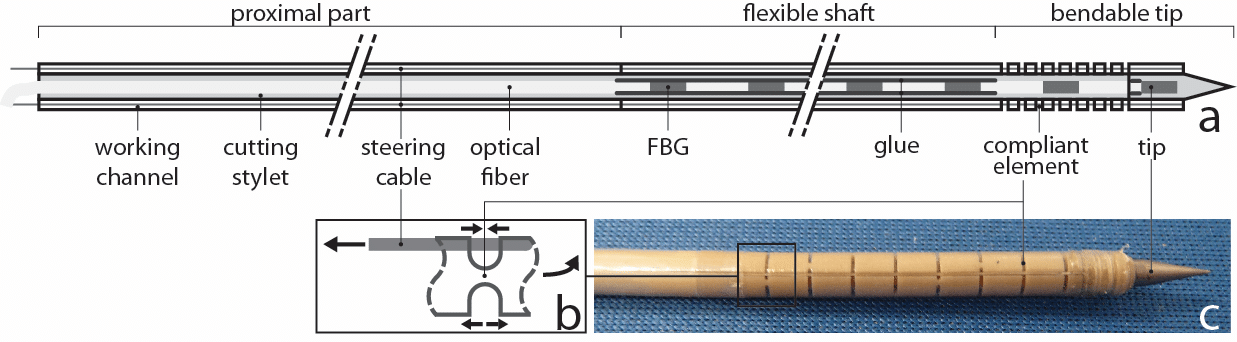
Figure 2 presents a schematic view of a steerable needle for which steerability is based on a higher compliancy near the tip. The needle can be divided in three parts in longitudinal direction:
- The proximal part relays the steering wires for actuation of the tip.
- The passively flexible shaft: the curvature of this passively flexible shaft is not controlled directly, but follows the direction of insertion introduced by the needle tip.
- The bendable tip: compliant hinges are integrated in the bendable tip to allow for the initiation of steering.
The degree and direction of bending of the tip can be adjusted by pulling one or two of the steering cables that run through the wall of the needle and are attached to the distal end of the needle. All materials used are MR compatible: the currently proposed concept makes use of PEEK, nitinol, Dynema, teflon and titanium (tip of the cutting stylet). Optionally the system can incorporate other devices for local measurements such as optical fibers for spectroscopy (i.e. for tissue characterisation) or shape sensing (i.e. for tracking of the needle tip).
An alternative approach for active needle steering is illustrated in Fig. 3.
The needle contains a cable driven ball joint near the tip that is actuated by a set of four wires, working in complementary pairs. By translation of the wires the needle tip can rotate in 3D space with 2 orthogonal rotational degrees of freedom (DOF) in effect realizing an adjustable bevel orientation. The wired are rigidly fixed to a handle with 2 rotational degrees of freedom for orienting the tip, altogether providing a 2 DOF actuation mechanism.